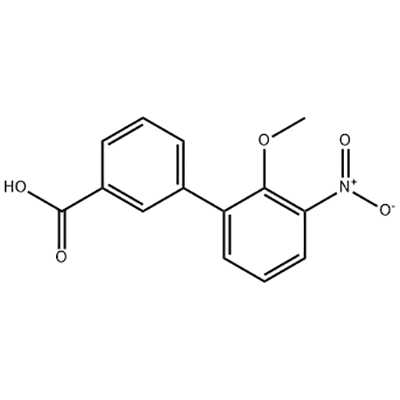
I-2'-Methoxy-3'-nitro-biphenyl-3-carboxylic acid
I-2'-Methoxy-3'-nitro-biphenyl-3-carboxylic acid
I-2'-Methoxy-3'-nitro-biphenyl-3-carboxylic acid isetshenziswa njengendawo emaphakathi ye-Eltrombopag.
I-Eltrombopag, eyakhiwe yi-GlaxoSmithKline (GSK) e-UK futhi kamuva yathuthukiswa ngokuhlanganyela ne-Novartis e-Switzerland, ingeyokuqala futhi iwukuphela kwe-molecule encane egunyazwe i-non-peptide TPO receptor agonist emhlabeni.I-Eltrombopag yagunyazwa yi-FDA yase-US ngo-2008 yokwelapha i-idiopathic thrombocytopenic purpura (ITP), nango-2014 ukuze kwelashwe i-aplastic anemia enzima (AA).Futhi isidakamizwa sokuqala esigunyazwe yi-US FDA sokwelapha i-AA eminyakeni engama-30 yakamuva.
Ngo-December 2012, i-US FDA yagunyaza i-Elthrombopag yokwelapha i-thrombocytopenia ezigulini ezine-hepatitis C (CHC) engapheli, ukuze iziguli ze-hepatitis C ezinokubikezelwa okungekuhle ngenxa yokubala kweplatelet ephansi zikwazi ukuqala futhi zilondoloze ukwelashwa okujwayelekile okusekelwe kwe-interferon kwezifo zesibindi.NgoFebhuwari3, 2014, i-GlaxoSmithKline yamemezela ukuthi i-FDA inikeze ukuphumelela komuthi wokwelapha i-Eltrombopag wokwelapha i-hemopenia ezigulini ezine-chemicalbook aplastic anemia (SAA) enzima kakhulu engazange iphendule ngokugcwele ku-immunotherapy.Ngomhla zingama-24 ku-Agasti, i-2015, i-US FDA igunyaze i-Eltrombopag yokwelashwa kwe-thrombocytopenia kubantu abadala kanye nezingane ezineminyaka engu-1 nangaphezulu ezine-immune immune thrombocytopenia (ITP) ezingenampendulo eyanele ku-corticosteroids, i-immunoglobulins noma i-splenectomy.NgoJanuwari4, 2018, i-Eltrombopag yagunyazwa ukuthi ifakwe ohlwini e-China ukuze zelashwe i-immune immune thrombocytopenia (ITP).



![i-pentamethylene bis[1-(3,4-dimethoxybenzyl)-3,4-dihydro-6,7-dimethoxy-1H-isoquinoline-2-propionate], i-dioxalate](http://cdn.globalso.com/jindunchem-med/image281-300x300.png)

![I-Casp ungin Acetate;Caspofungin acetate;Cancidas;Caspofungin acetate [USAN:BAN:JAN];](http://cdn.globalso.com/jindunchem-med/fbe17385-300x300.jpg)