I-Factory Supply 4-Bromo-2-Fluorobenzoic Acid 4-Bromo-2-Fluorobenzoic Acid CAS 112704-79-7
I-Factory Supply 4-Bromo-2-Fluorobenzoic Acid 4-Bromo-2-Fluorobenzoic Acid CAS 112704-79-7
Ukuze sigcwalise ukwaneliseka obekulindeleke kakhulu kumakhasimende , manje sineqembu lethu eliqinile elizohlinzeka ngosizo lwethu oluvamile oluhlanganisa ukuphromotha, ukuthengisa okuphelele, ukuhlela, ukudala, ukulawulwa kwekhwalithi ephezulu, ukupakisha, ukugcinwa kwempahla kanye nokusetshenziswa kweFactory Supply 4-Bromo-2 -Fluorobenzoic Acid 4-Bromo-2-Fluorobenzoic Acid CAS 112704-79-7, Siqotho futhi sivulekile.Sibheke phambili ekuvakasheni kwakho nokusungula ubudlelwano obuthembekile nobuhlala isikhathi eside.
Ukuze sigcwalise ukwaneliseka obekulindeleke kakhulu kumakhasimende , manje sineqembu lethu eliqinile elizohlinzeka ngosizo lwethu olujwayelekile oluhlanganisa ukuphromotha, ukuthengisa okuphelele, ukuhlela, ukudala, ukulawula ikhwalithi ephezulu, ukupakisha, ukugcinwa kwempahla kanye nokuhlelwa kwempahla.I-China CAS 112704-79-7 kanye ne-112704-79-7, Izimpahla zethu zizuze idumela elihle kakhulu emazweni ahlobene.Ngoba ukusungulwa kwefemu yethu.sigcizelele ekusunguleni inqubo yethu yokukhiqiza kanye nendlela yakamuva yokuphatha yesimanje, eheha inani elikhulu lamathalente kulo mkhakha.Sithatha isixazululo sekhwalithi enhle njengomlingiswa wethu obaluleke kakhulu.
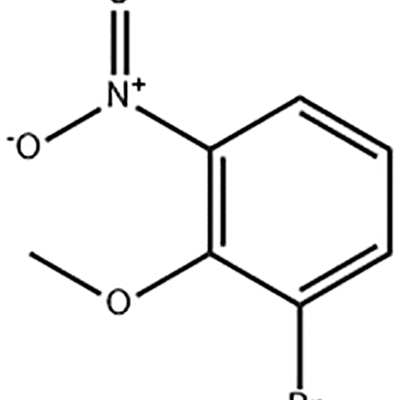
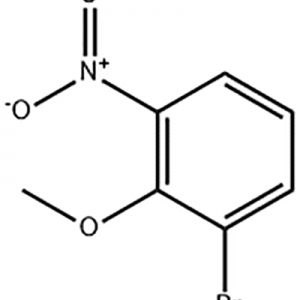
I-1-Bromo-2-methoxy-3-nitro-benzene isetshenziswa njengendawo emaphakathi ye-Eltrombopag .
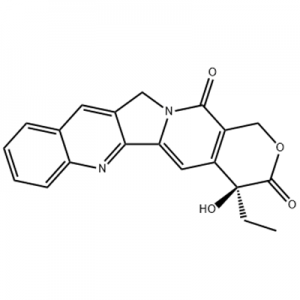
I-Eltrombopag, eyakhiwe yi-GlaxoSmithKline (GSK) e-UK futhi kamuva yathuthukiswa ngokuhlanganyela ne-Novartis e-Switzerland, ingeyokuqala futhi iwukuphela kwe-molecule encane egunyazwe i-non-peptide TPO receptor agonist emhlabeni.I-Eltrombopag yagunyazwa yi-FDA yase-US ngo-2008 yokwelapha i-idiopathic thrombocytopenic purpura (ITP), nango-2014 ukuze kwelashwe i-aplastic anemia enzima (AA).Futhi isidakamizwa sokuqala esigunyazwe yi-US FDA sokwelapha i-AA eminyakeni engama-30 yakamuva.
Ngo-December 2012, i-US FDA yagunyaza i-Elthrombopag yokwelapha i-thrombocytopenia ezigulini ezine-hepatitis C (CHC) engapheli, ukuze iziguli ze-hepatitis C ezinokubikezelwa okungekuhle ngenxa yokubala kweplatelet ephansi zikwazi ukuqala futhi zilondoloze ukwelashwa okujwayelekile okusekelwe kwe-interferon kwezifo zesibindi.NgoFebhuwari3, 2014, i-GlaxoSmithKline yamemezela ukuthi i-FDA inikeze ukuphumelela komuthi wokwelapha i-Eltrombopag wokwelapha i-hemopenia ezigulini ezine-chemicalbook aplastic anemia (SAA) enzima kakhulu engazange iphendule ngokugcwele ku-immunotherapy.Ngomhla zingama-24 ku-Agasti, i-2015, i-US FDA igunyaze i-Eltrombopag yokwelashwa kwe-thrombocytopenia kubantu abadala kanye nezingane ezineminyaka engu-1 nangaphezulu ezine-immune immune thrombocytopenia (ITP) ezingenampendulo eyanele ku-corticosteroids, i-immunoglobulins noma i-splenectomy.NgoJanuwari4, 2018, i-Eltrombopag yagunyazwa ukuthi ifakwe ohlwini e-China ukuze zelashwe i-immune immune thrombocytopenia (ITP).
Ukuze sigcwalise ukwaneliseka obekulindeleke kakhulu kumakhasimende , manje sineqembu lethu eliqinile elizohlinzeka ngosizo lwethu oluvamile oluhlanganisa ukuphromotha, ukuthengisa okuphelele, ukuhlela, ukudala, ukulawulwa kwekhwalithi ephezulu, ukupakisha, ukugcinwa kwempahla kanye nokusetshenziswa kweFactory Supply 4-Bromo-2 -Fluorobenzoic Acid 4-Bromo-2-Fluorobenzoic Acid CAS 112704-79-7, Siqotho futhi sivulekile.Sibheke phambili ekuvakasheni kwakho nokusungula ubudlelwano obuthembekile nobuhlala isikhathi eside.
Ukunikezwa KwemboniI-China CAS 112704-79-7 kanye ne-112704-79-7, Izimpahla zethu zizuze idumela elihle kakhulu emazweni ahlobene.Ngoba ukusungulwa kwefemu yethu.sigcizelele ekusunguleni inqubo yethu yokukhiqiza kanye nendlela yakamuva yokuphatha yesimanje, eheha inani elikhulu lamathalente kulo mkhakha.Sithatha isixazululo sekhwalithi enhle njengomlingiswa wethu obaluleke kakhulu.